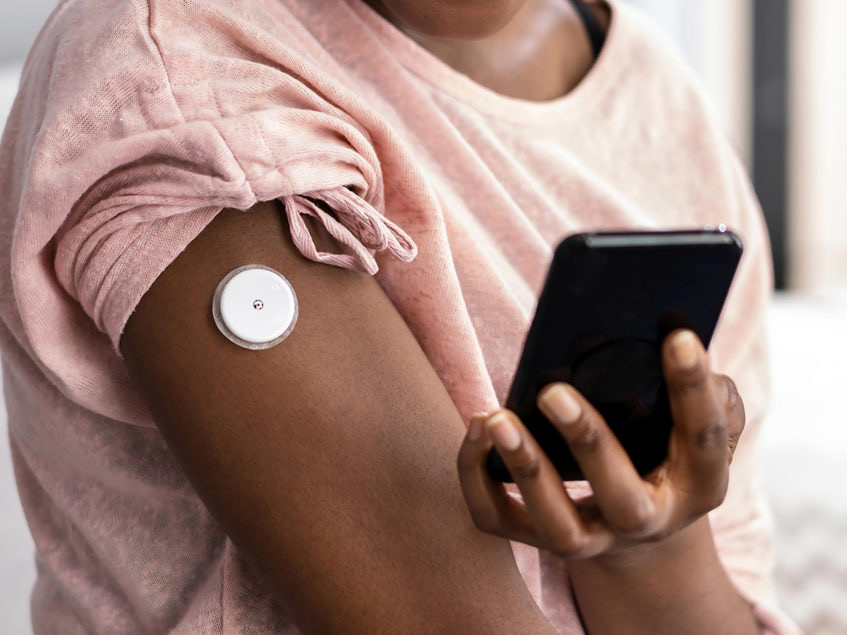
The US Food and Drug Administration has formally approved Abbott’s two new over-the-counter continuous glucose monitoring (CGM) systems in Lingo™ and Libre Rio™. Based on the company’s world-leading FreeStyle Libre® continuous glucose monitoring technology, these monitoring systems are designed to serve a different purpose in their own right. For instance, while Lingo is meant for consumers who want to better understand and improve their health and wellness, Libre is geared towards those adults with Type 2 diabetes who do not use insulin and typically manage their diabetes through lifestyle modifications. Talk about these devices on a slightly deeper level, we begin from Lingo, which can be used by consumers 18 years and older. For this market, Lingo will track glucose and provide personalized insights, along with customized coaching to help people create healthy habits, retrain their metabolism and improve their overall well-being. As for how it works, the monitoring system basically leverages a biosensor, which is to beworn on the upper arm for 14 days, and continuously streams glucose data to a coaching application on a smart phone, data that depicts the body’s language and gives out insights on the person’s reaction to food, exercise, and life’s daily stressors.
To understand the significance of such a development, though, we must take a look at one study done by the University of North Carolina. This study would go on to reveal how only 12 percent of Americans are metabolically healthy based on five key indicators of metabolic health, including glucose levels, proving that most of the U.S. population has room to improve their metabolism. Contextualizing the given significance even more is a separate survey online consumer survey conducted by The Harris Poll on behalf of Abbott. Here, it was revealed that more than 82 percent of Americans say they would change their habits if a biowearable provided actionable, personalized coaching to help them manage their health.
“Continuous glucose monitors are a tool I recommend to my patients to raise their overall awareness of factors that affect their glucose and are an invaluable holistic wellness solution,” said Fred St. Goar, M.D., cardiologist and medical director of El Camino Health Heart and Vascular Institute. “Research has shown that overall lower glucose exposure in the general population is associated with reduced long-term risk to developing cardiovascular disease, diabetes, Alzheimer’s and certain cancers. Making continuous glucose monitors widely available will undoubtedly have a dramatic effect on the overall health and well-being of the broader population.”
Turning our attention towards Libre Rio, it happens to be Abbott’s first over-the-counter CGM system for people with diabetes in the U.S. Not just that, it also happens to be the first ever over-the-counter CGM system with a measurement range of 40-400 mg/dL which allows for measurement of extremely low or high glucose events. Libre Rio has its importance rooted in the fact that diabetes is among the top public health challenges across US, with approximately 38.4 million people currently suffering from the condition. Hence, to serve such a big faction of people, the device will join Abbott’s overall Libre portfolio of CGM systems, which is now used by about 6 million people in more than 60 different countries. The stated portfolio markedly comprises of FreeStyle Libre 2 and FreeStyle Libre 3 systems for people with all types of diabetes, including Type 1, Type 2, and gestational. Requiring a prescription for use, these systems also have wide reimbursement by most major insurers in the U.S. This proposition, of course, is only made better by extensive clinical and real-world data from the millions of people who use FreeStyle Libre systems. This data shows us how the technology has already helped people improve their glucose control, lower their HbA1c, decrease diabetes-related hospital admissions, and improve their quality of life.